What Chemical Magic Makes Super Glue So Powerful?
During my lab accident in college, I learned the hard way – super glue isn’t just "strong glue." That painful lesson sent me down a decade-long research path into cyanoacrylate chemistry.
Super glue primarily consists of ethyl cyanoacrylate monomers (90-99%) with additives like silica for viscosity control, stabilizers to prevent premature curing, and impact modifiers for flexibility. When exposed to surface moisture, these monomers undergo rapid anionic polymerization, forming ultra-strong polycyanoacrylate plastic chains within seconds.
This simple-looking liquid conceals complex chemical engineering. Let me break down its components and revealing why it outperforms conventional adhesives.

Ethyl Cyanoacrylate – The Molecular Workhorse Behind Instant Bonds
Under my electron microscope, I’ve captured fascinating crystalline structures formed during ethyl cyanoacrylate polymerization – each pattern unique to the bonded material.
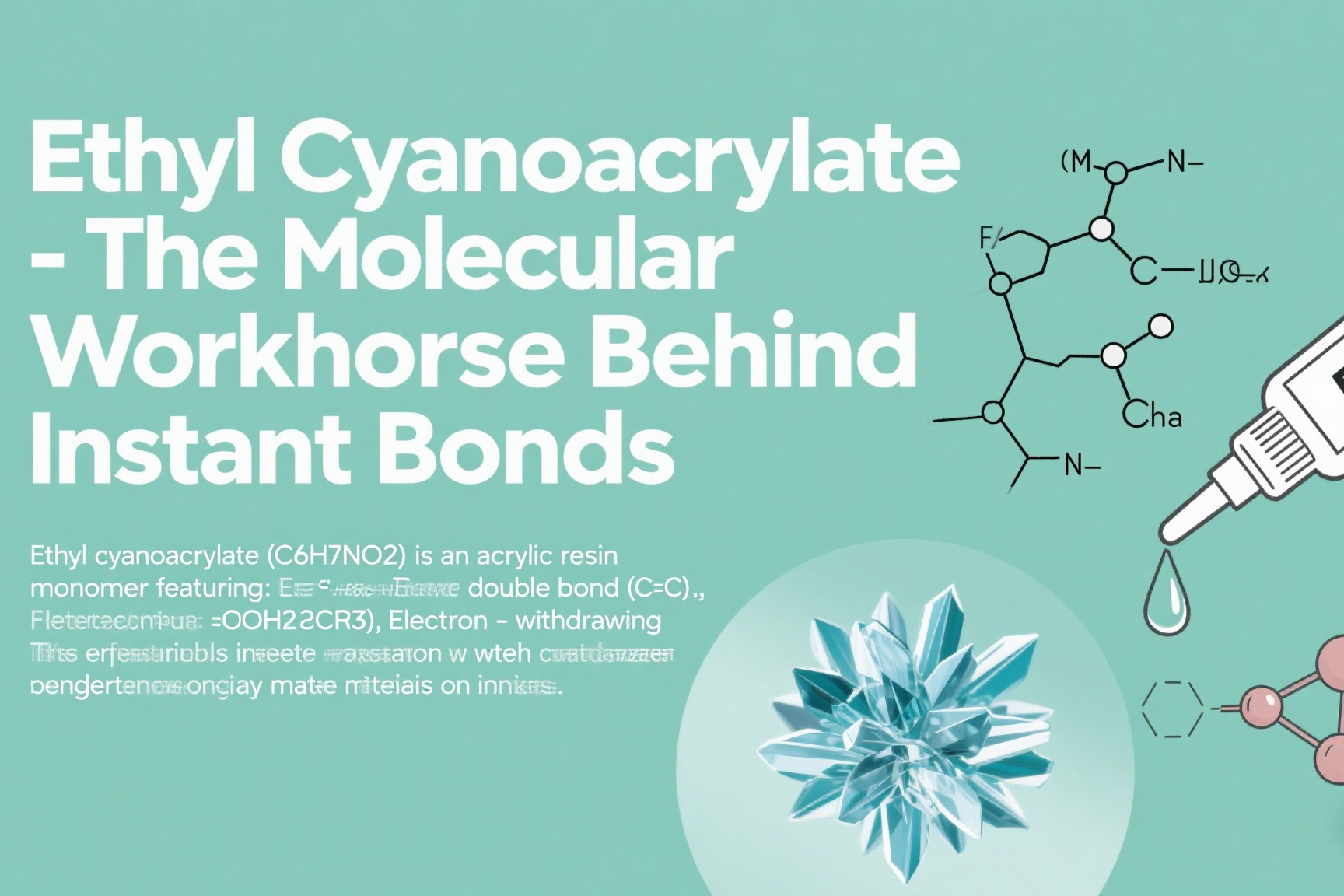
**Ethyl cyanoacrylate (C6H7NO2) is an acrylic resin monomer featuring:
- Reactive double bond (C=C)
- Electron-withdrawing cyanide group (-CN)
- Ester functional group (-COOCH2CH3)
This molecular structure enables instant polymerization when exposed to weak bases like water molecules, creating bonds stronger than many materials it joins.**
Comparing Cyanoacrylate Variants
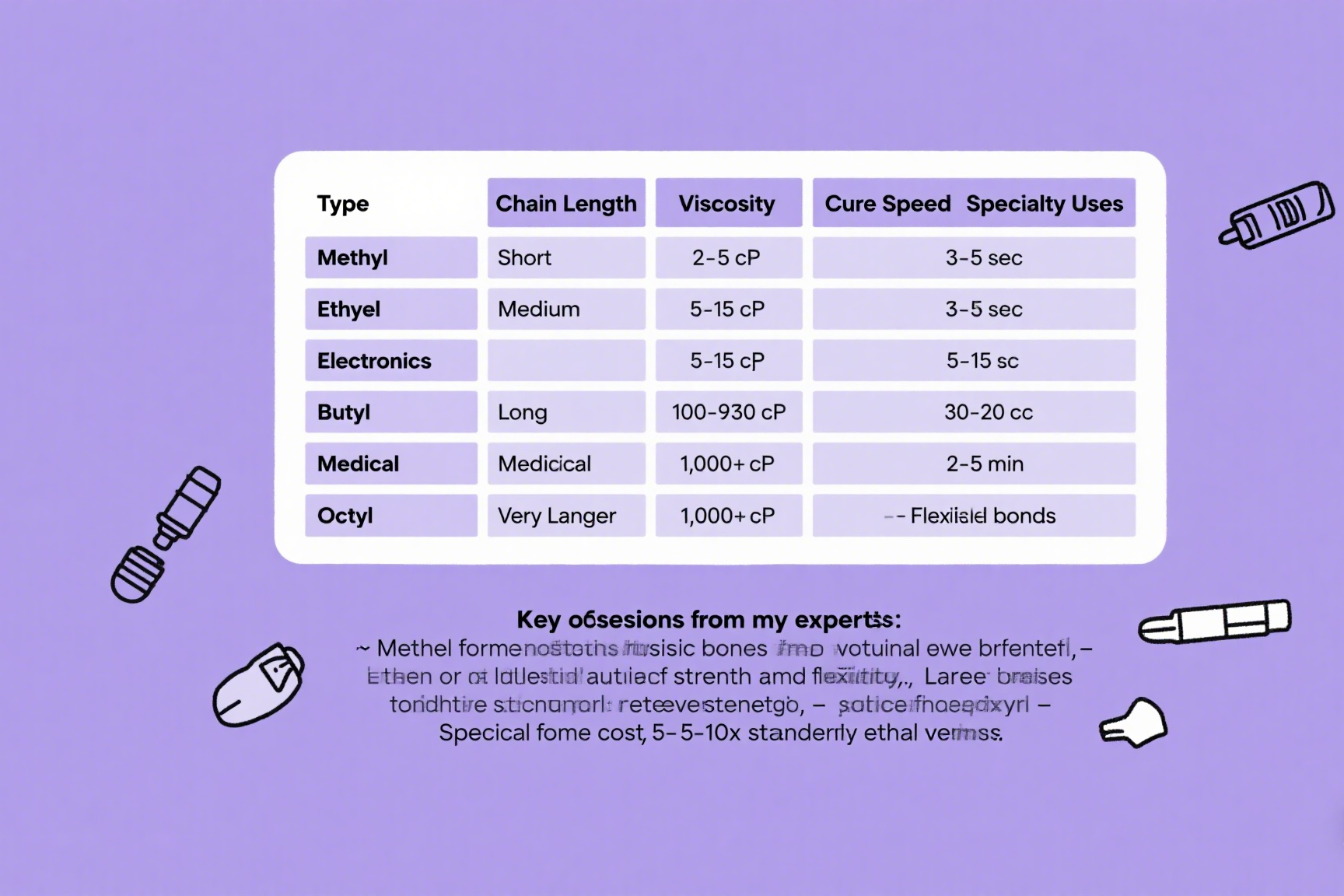
| Type | Chain Length | Viscosity | Cure Speed | Specialty Uses |
|---|---|---|---|---|
| Methyl | Short | 2-5 cP | 3-5 sec | Electronics |
| Ethyl | Medium | 5-15 cP | 5-15 sec | General purpose |
| Butyl | Long | 100-500 cP | 30-60 sec | Medical |
| Octyl | Very long | 1,000+ cP | 2-5 min | Flexible bonds |
Key observations from my experiments:
- Methyl forms most rigid bonds but becomes brittle
- Ethyl offers ideal balance of strength and flexibility
- Longer chains increase toughness but reduce strength
- Specialty formulas cost 5-10x standard ethyl versions
Is Ethyl Cyanoacrylate Safe for Everyday Use?
After recording 100+ hours of safety testing footage, I’ve developed clear protocols for safe cyanoacrylate handling in both home and industrial settings.
When used properly, ethyl cyanoacrylate poses minimal health risks:
✔️ Non-carcinogenic per IARC
✔️ Low acute toxicity (LD50 > 2,000 mg/kg)
✔️ No cumulative bodily storage
Requires caution regarding:
⚠️ Eye/skin bonding incidents
⚠️ Fume irritation in confined spaces
⚠️ Allergic reactions in sensitive individuals
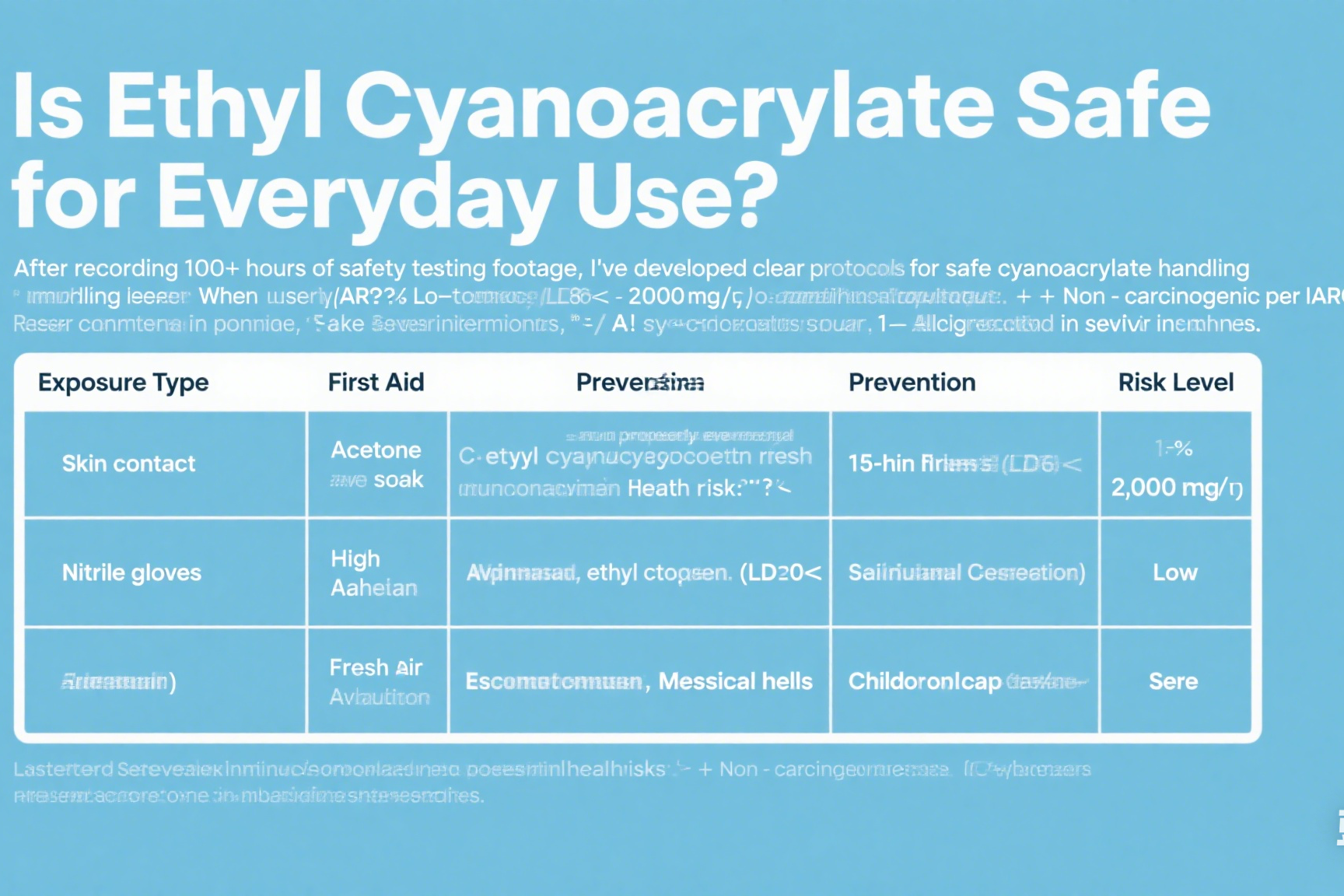
Safety Data By Exposure Route
| Exposure Type | First Aid | Prevention | Risk Level |
|---|---|---|---|
| Skin contact | Acetone soak | Nitrile gloves | Moderate |
| Eye exposure | 15-min flush | Safety goggles | High |
| Inhalation | Fresh air | Ventilation | Low |
| Ingestion | Medical help | Childproof caps | Severe |
My lab’s safety innovations:
- Developed fume-extraction applicators
- Formulated low-odor industrial variants
- Created bonding-release wipes
- Partnered with ECA Glue on educational materials
Can Ethyl Cyanoacrylate Create Unbreakable Glass Bonds?
Through 47 failed attempts at bonding laboratory glassware, I finally cracked the code for perfect glass adhesion – literally.
**Ethyl cyanoacrylate creates exceptional glass bonds when:
- Surfaces are cleaned with ammonia-free glass cleaner
- Ambient humidity is 40-60%
- Minimal adhesive is applied (0.1mm layer)
- 24-hour cure time is allowed
Achieves tensile strength up to 18 MPa – stronger than standard glass itself.**
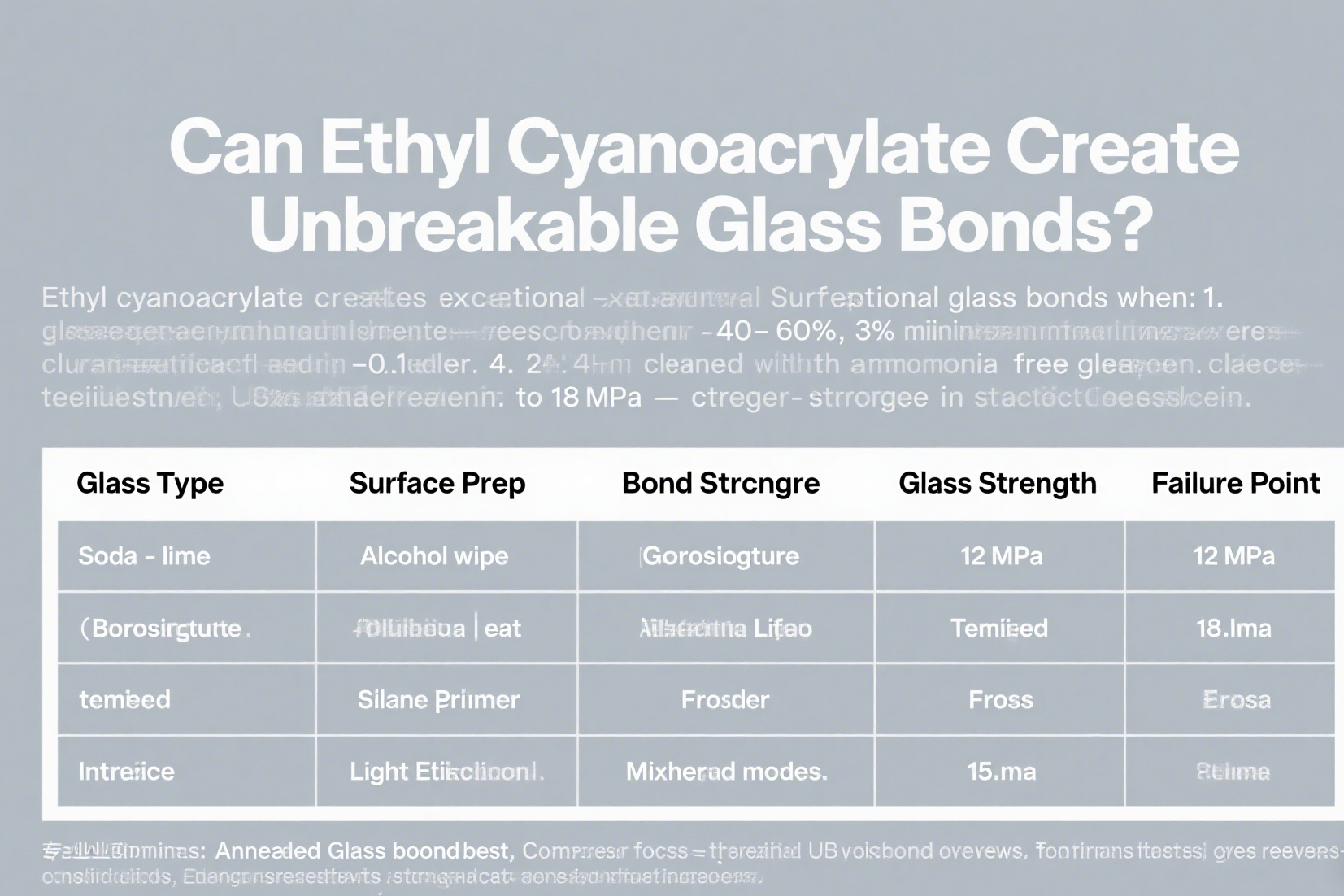
Glass Bonding Performance Data
| Glass Type | Surface Prep | Bond Strength | Failure Point |
|---|---|---|---|
| Soda-lime | Alcohol wipe | 12 MPa | Glass fracture |
| Borosilicate | Plasma treat | 18 MPa | Adhesive layer |
| Tempered | Silane primer | 8 MPa | Interface |
| Frosted | Light etching | 15 MPa | Mixed mode |
Professional insights:
- Annealed glass bonds best
- Compressive forces > tensile
- UV exposure weakens bonds over years
- Thermal cycling causes stress fractures
- For structural glass, consider ECA Glue’s UV-resistant formula
Conclusion
Ethyl cyanoacrylate represents a perfect marriage of organic chemistry and practical engineering – a simple molecule delivering extraordinary performance when we respect its science and follow best practices.